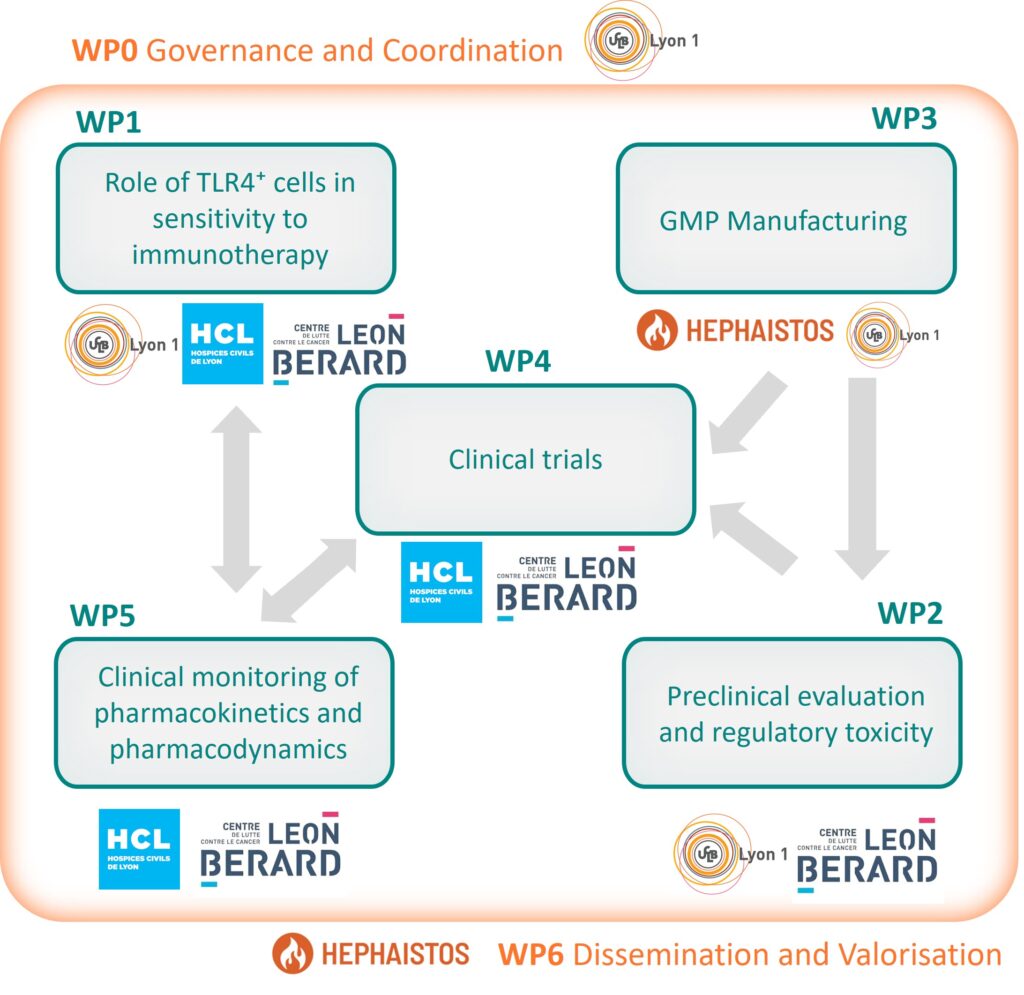
WP0
Coordination and Governance

This ambitious project involves numerous stakeholders. To ensure the flow of information within the consortium and meet the requirements of the various sectors involved (academic, medical, financial, regulatory, ethical), rigorous management and coordination are essential. In order to ensure the best possible direction for the SyStInn project, its governance is entrusted to a strategic committee, a steering committee, and an scientific advisory board. This project must also comply with the requirements and timeline set by the funding agency, the ANR. This includes the submission of annual scientific and financial reports, the organization of annual follow-up meetings, and the development of a data management plan.
WP1
Role of TLR4+ cells in sensitivity to immunotherapy

TLR4 is the receptor of HEPHA-440. It is primarily expressed by certain cells of the innate immune system such as monocytes, macrophages, and dendritic cells. The goal of the research is to elucidate the mechanisms of action of HEPHA-440 at the molecular and cellular levels and to understand the role of cells expressing the TLR4 receptor in the response to immunotherapy. While adaptive immune memory, which allows lymphocytes to respond specifically and rapidly if an invader reappears, is well-known and described, the hypothesis of innate immune memory is emerging and will be explored in this context.
WP2
Preclinical evaluation and regulatory toxicity

This part aims to characterize the antitumor efficacy of HEPHA-440 alone and in combination with other immunotherapies, as well as its tolerability, in animal models (mice and rabbits). The regulatory preclinical toxicology study will be conducted by an external company according to Good Laboratory Practices (GLP), in order to understand the fate of the drug in the body and characterize its effects on various organs. This is a key element for drug safety agencies to approve the first human administrations.
WP3
GMP Manufacturing

This phase, managed by the industrial partner, aims to manufacture the batches of HEPHA-440 intended for clinical use, ensuring that a number of quality criteria (purity, batch reproducibility, etc.) are met in accordance with Good Manufacturing Practices (GMP). The stability of the compound will also be evaluated to determine its packaging and shelf life. In preparation for a potential market launch at the end of the project, large-scale production of HEPHA-440 will then be required.
WP4
Clinical trials

The clinical trials aim to study the safety and efficacy of HEPHA-440 in individuals with cancer. During Phase I, the drug will be administered at increasing doses to adults with terminal cancer. All of its effects on the body will be investigated, allowing for the determination of a safe and effective dose for Phase II, known as the RP2D (Recommended Dose for Phase II trial). Two Phase II trials will then be conducted. The efficacy of the drug alone at the RP2D will be evaluated in 18 patients with osteosarcoma, a bone cancer primarily affecting children and adolescents. In parallel, the efficacy of HEPHA-440 in combination with other immunotherapies (immune checkpoint inhibitors) will be tested on a cohort of 25 patients with various solid tumors.
WP5
Clinical monitoring of pharmacokinetics and pharmacodynamics
Through blood samples collected from patients included in the clinical trials, the biological effects of HEPHA-440 will be studied. One primary objective will be to elucidate the mechanisms of action, particularly on the immune system, of HEPHA-440 (pharmacodynamics). Another aspect will be to characterize the distribution of HEPHA-440 in the body (pharmacokinetics). Statistical analysis of the various clinical data will help define efficacy markers, with the ultimate goal of identifying patients who are likely to respond to the treatment.
WP6
Dissemination and Valorisation

Throughout the project, discussions will take place with the entire consortium regarding the management of intellectual property for the results obtained. These may be kept confidential, disseminated, protected by a patent, or transferred to third parties. The results we decide to share will be communicated through regular updates on the website, published in international scientific journals, and presented at conferences. Furthermore, the data obtained during the clinical trials will be made available on the clinicaltrials.gov. Finally, if the clinical data confirm the safety and efficacy of the treatment, steps will be taken for its market authorization.